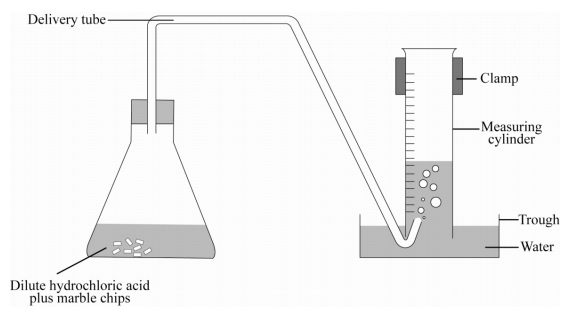
With the equation caco3 2hcl cacl2 h2o co2 hypotheses a reaction occurs when particles collide.
Marble chips and hydrochloric acid.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
This process is based on random particle movement.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
Marble chips and hydrochloric acid planning aim to find if changing the concentration of an acid will increase or decrease the rate of the reaction when marble is dissolved in hydrochloric acid.